Defective DePuy ASR XL Hips
 Johnson and Johnson's DePuy subsidiary recalled its ASR XL Acetabular hip replacement implant devices affecting over 93,000 patients. Our firm is now accepting DePuy hip implant recipients across the United States who are experiencing pain, discomfort or who had a revision surgery to replace the defective device. If you had hip replacement surgery and your doctor implanted a DePuy ASR XL device and you either had revision surgery or you've been experiencing pain and discomfort with the device, we want to talk with you. The recall may entitle you to substantial monetary damages. We practice in the state and federal courts across the State of Alabama and we're accepting clients statewide that are dealing with this recall. We have a track record of success representing the injured in personal injury and product liability cases. Call us to discuss your situation and allow us to evaluate your potential claims. The consultation is free.
Johnson and Johnson's DePuy subsidiary recalled its ASR XL Acetabular hip replacement implant devices affecting over 93,000 patients. Our firm is now accepting DePuy hip implant recipients across the United States who are experiencing pain, discomfort or who had a revision surgery to replace the defective device. If you had hip replacement surgery and your doctor implanted a DePuy ASR XL device and you either had revision surgery or you've been experiencing pain and discomfort with the device, we want to talk with you. The recall may entitle you to substantial monetary damages. We practice in the state and federal courts across the State of Alabama and we're accepting clients statewide that are dealing with this recall. We have a track record of success representing the injured in personal injury and product liability cases. Call us to discuss your situation and allow us to evaluate your potential claims. The consultation is free.
Doyle Law is now accepting clients who had hip replacement implants manufactured by DePuy Orthopaedics, Inc., that are subject to the recent Class 2 Recall announced by the FDA on March 24, 2011. If you had a DePuy ASR XL Acetabluar Cup System installed during hip replacement surgery between 2003 and 2010, contact Alabama-licensed product liability trial attorney Jimmy Doyle to discuss your potential claims. We are evaluating hip replacement complications in the DePuy implant and we've begun filing lawsuits nationwide.

DePuy Hip Recall Lawyers
In September of 2010, DePuy Orthopaedic, a division of Johnson and Johnson, announced their intent to conduct a recall of its ASR XL Acetabular System and ASR Hip Resurfacing Platforms. The hip implant recall was spurred by data that showed after hip replacement surgery, a revision surgery rate of about 13 percent for those with the ASR XL system and of about 12 percent for those with the ASR resurfacing system.
The DePuy ASR recalled devices have caused their recipients to suffer chronic pain and difficulty walking due to the defective design. Based on the data provided to the FDA, the Johnson and Johnson DePuy ASR recall will affect approximately 93,000 current DePuy implants.
Manufacturers are required by law to practice certain safety requirements and quality control measures to ensure that the products they produce are safe for public use. If a personal injury occurs due to product defects, manufactures can be held liable for the damages their defective products cause.
The recall on hip replacement implant devices by DePuy is only one of several that have occurred recently. Both Zimmer and Stryker have also issued hip replacement recalls of several of their devices. Please visit our Zimmer Page for information on that recall if you had a Durom Cup device installed.
Which DePuy Devices Recalled?
DePuy Orthopaedics, Inc., acquired by Johnson & Johnson, issued a voluntary recall of its ASR XL Acetabular System and ASR Hip Resurfacing Platforms that were implanted after July 2003. The DePuy hip recall doesn not apply to patients who had artificial hip surgery prior to July 2003; those patients and hip replacement implant devices are not affected by the recall.
DePuy disclosed that hip replacement complications related to their implants produced on or after July 2003 were the reason for its recall. The hip recall became necessary because the unexpected number of complaints of pain and other symptoms by patients who have received either a total hip replacement or an ASR Hip Resurfacing Platform. In addition, DePuy states that their studies have shown that 1 in 8 patients who received the ASR resurfacing device required a second (revision) surgery, while 1 in 8 of patients who received the total hip replacement surgery required a revision surgery. That is well above the acceptable failure rate - and that ratio continues to rise.
Possible Complications of DePuy Hip Implants
The defective design of the ASR hip replacement cup, which is shallower than similar hip replacement devices, is responsible for many of the product’s problems. The ASR hip replacement cup doesn’t always affix to the bone as intended. Too often, this results in a "loose cup" that must be revised, or removed and replaced through a corrective surgery (aka, "revision surgery").
The DePuy ASR hip replacement implant is a metal-on-metal hip replacement system. These defective hip implants can shed metal particles and debris into the body as they wear. That debris can cause severe inflammatory reactions in some patients, damaging muscles and soft tissue and lead to bone loss. As a result, a second corrective hip replacement surgery is often required to replace the defective hip device soon after implant.

Contact Alabama DePuy Hip Replacement Recall Attorneys
If you've encountered problems with your DePuy device during your hip replacement recovery or if you have encountered pain or a loosening of your DePuy-Johnson and Johnson ASR device since surgery - and that is subject to the DePuy hip implant recall - a DePuy hip lawsuit may be a viable option for you. Call our Birmingham, Alabama offices today for a free consultation. The number to reach either office is (205) 533-9500. Do not wait another day and risk losing your claim.
The attorneys at the Doyle Law Firm are now filing DePuy hip lawsuits for clients and are evaluating DePuy recall hip claims nationwide. If you have one of the defective ASR devices subject to the 2011 hip replacement recall, you may be entitled to monetary compensation for your pain and suffering through a DePuy lawsuit.
The Doyle Law Firm's experienced injury lawyers have extensive experience with product liability claims, specifically those involving defective medical devices. If you have received a DePuy implant since 2003, call us today even if you're not sure of your implant model. We can help you to identify your device and determine whether a DePuy hip replacement lawsuit makes sense for you.
Alabama has a two year statute of limitations on filing certain product liability and personal injury claims. It takes time to acquire medical records and draft pleadings, so please do not delay in contacting us if you have any concern that your hip replacement device may be subject to this recall.

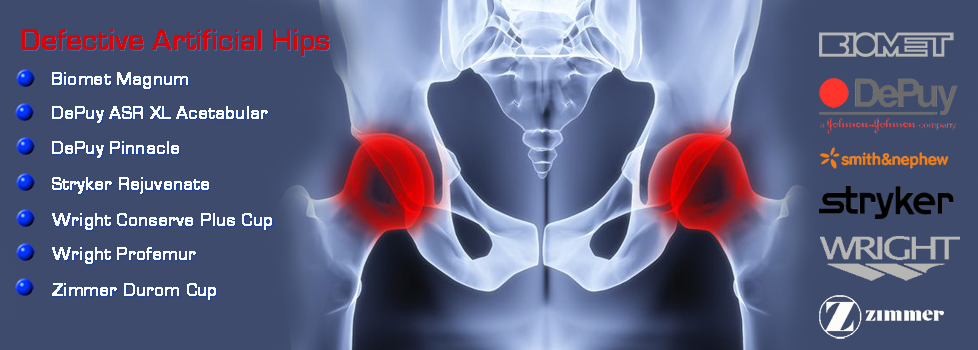


 Fosamax, Boniva, Actonel, Aredia, and Zometa - medication prescribed to treat osteoporosis - are linked to femur fractures after 3 years of use. If you took any of this medication (or any combination thereof) and suffered a femur fracture,
Fosamax, Boniva, Actonel, Aredia, and Zometa - medication prescribed to treat osteoporosis - are linked to femur fractures after 3 years of use. If you took any of this medication (or any combination thereof) and suffered a femur fracture,  Mesh used during surgery to repair the vaginal wall has caused women to experience mesh erosion into the vagina, prolapse, pain during intercourse, incontinence, bowel or bladder perforation, bleeding and serious infection. We're now accepting clients with claims against AMS, Bard, Boston Scientific, Ethicon, and Johnson & Johnson. If you took have suffered any of these side effects,
Mesh used during surgery to repair the vaginal wall has caused women to experience mesh erosion into the vagina, prolapse, pain during intercourse, incontinence, bowel or bladder perforation, bleeding and serious infection. We're now accepting clients with claims against AMS, Bard, Boston Scientific, Ethicon, and Johnson & Johnson. If you took have suffered any of these side effects,  A growing number of metal-on-metal artificial hip implants are being recalled due to early failures and some patients suffering from metallosis - metal debris released from the hip compontents inflame the surrounding tissue and enter the bloodstream, causing elevated levels of cobalt and chromium. We're now accepting clients with claims aginst Biomet, DePuy, Stryker, Smith & Nephew, Wright, and Zimmer. If you have (or had) one of these devices, we can help to protect your legal rights. To contact us,
A growing number of metal-on-metal artificial hip implants are being recalled due to early failures and some patients suffering from metallosis - metal debris released from the hip compontents inflame the surrounding tissue and enter the bloodstream, causing elevated levels of cobalt and chromium. We're now accepting clients with claims aginst Biomet, DePuy, Stryker, Smith & Nephew, Wright, and Zimmer. If you have (or had) one of these devices, we can help to protect your legal rights. To contact us, 