Paxil Birth Defects
 Paxil is a drug that has recently been linked to birth defects after use during pregnancy. A variety of potential birth defects have been documented by mothers that used the prescription medication during pregnancy, especially during the first trimester. If your baby was born with a birth defect after your Paxil use, we want to speak with you.
Paxil is a drug that has recently been linked to birth defects after use during pregnancy. A variety of potential birth defects have been documented by mothers that used the prescription medication during pregnancy, especially during the first trimester. If your baby was born with a birth defect after your Paxil use, we want to speak with you.
We are representing families across the State of Alabama and we're still accepting new clients. In those cases where we can establish Paxil use during pregnancy, we are filing claims on behalf of families affected by this defective drug. We'll review your case and closely evaluate the potential product liability claims involving Paxil. If you took this drug to treat depression, OCD, panic attacks, social anxiety disorders, or PTSD and your child was born with a birth defect, you should not wait another day to contact us. Paxil is manufactured by global health care company GlaxoSmithKline, Inc.
What is Paxil?
Paxil® is generically known as paroxetine and is a drug prescribed to treat major depression in adults, as well as obsessive-compulsive disorder, panic and social anxiety disorders and post-traumatic stress disorder in both adults and children. Paxil is classified as a selective serotonin reuptake inhibitor (SSRI) drug, meaning that it selectively affects serotonin. Serotonin is one of many chemicals in the brain called neurotransmitters, which pass messages between nerve cells, and has recently been linked in various studies to an increased risk of birth defects. Recent studies have shown that serotonin, even during the first trimester of pregnancy, helps to shape the basic brian function that will be applied to learning and emoting later in life.

Paxil Side Effects
The Food and Drug Administration (FDA) initially placed SSRI antidepressants, including Paxil, in its pregnancy "Category C," which means that animal reproduction studies have shown an adverse effect on the fetus. However, there have not yet been adequate and well-controlled studies in humans. Pregnancy categories measure the teratogenic effects a drug has on a fetus. Teratogenic means that a drug or other substance is capable of interfering with the development of a fetus, so there could be serious risks to the unborn baby of a woman taking Paxil while pregnant.
Birth defects or conditions that may be associated with the use of Paxil include:
- Abdominal wall defects (infant omphalocele)
- Anal atresia (complete or partial closure of the anus)
- Cleft lip
- Cleft palate
- Heart defects
- Skull defect (craniosynostosis)
- Clubfoot (one or both feet turn downward and inward)
- other malformations
What Dangers Are Associated With Paxil?
Although Paxil had shown significant promise in treating multiple disorders, complaints have surfaced over time related to the use of Paxil during pregnancy. Of course, some side effects can be expected from the use of powerful medications like Paxil; however, the issues cited by some Paxil users have been far more serious than the mild side effects outlined in the drug’s labeling.
Most notably, women who used Paxil during pregnancy began to show extremely high rates of serious birth defects, like cleft lip and cleft palate in their children. Such a warning is nowhere to be found in the label, which raises serious questions about the testing and scientific research GlaxoSmithKline undertook during product development. And, when GlaxoSmithKline rushed this drug through the FDA to the marketplace, it is likely that they disregarded scientific consensus about SSRI effects - in order to make a hefty profit.
In September 2005, drug manufacturer GlaxoSmithKline informed health care professionals that it was updating the Pregnancy subsection of the PRECAUTIONS section of the labels for the drug PAXIL (paroxetine HCI) and PAXIL CR controlled-release tablets.
The new warning reflects the results of a study that indicates major congenital malformations in infants born to women taking antidepressants, including PAXIL, during the first trimester of pregnancy. According to an FDA MedWatch Alert, the study shows an increased risk of overall major congenital malformations for paroxetine in particular, as compared to other antidepressants.
These findings are a result of a retrospective epidemiologic study of 3,581 pregnant women, conducted by GSK, which examined major congenital malformations in infants born to women taking antidepressants in the first trimester of pregnancy.
The study revealed a greater risk for overall major congenital malformations for women using paroxetine compared to other antidepressants, as well as an increased risk for cardiovascular malformation using paroxetine as compared to other antidepressants. Ten out of 14 infants with cardiovascular malformations had ventricular septal defects.
Autism and Antidepressants
The Archive of General Psychiatry published a report in July of 2011 linking SSRI antidepressants such as Paxil to a potentially increased risk of autism spectrum disorders (ASDs) in babies born to mothers who took these drugs during the first trimester of pregnancy. Performed by researchers at the Kaiser Permanente Medical Care Program in Northern California, the population-based, case-control study compared a group of 298 children having varying degrees of autism and their mothers to another group of 1,507 randomly selected children and their mothers. Nearly 70 children from each group were exposed to antidepressants in the same class as Paxil, and the researchers found a possible connection between mothers who took these drugs within a year before delivery and an increased the risk of their babies being born with ASDs. The highest risk was found to be among those whose mothers took these drugs during the first trimester.

Contact Alabama Paxil Birth Defect Attorneys
If your baby was born with a birth defect after your Paxil use during pregnancy, you may have a defective product claim against GlaxoSmithKline, Inc. Call our Birmingham, Alabama, offices today for a free consultation. Our office number is 205.533.9500. Do not wait another day and risk losing your claim.
The attorneys at the Doyle Law Firm are now accepting Paxil clients and filing lawsuits nationwide. Due to your baby's injury, you may be able to recover damages (monetary compensation) for your pain, suffering, and medical treatment expenses. The Doyle Law Firm's experienced birth defect trial lawyers have extensive experience with product liability claims, specifically those involving defective and dangerous drugs.
Alabama Paxil Statute of Limitations
Alabama has a two year statute of limitations on filing certain product liability and personal injury claims. It takes time to acquire medical records and draft pleadings, so please do not delay in contacting us if you have any concern that your baby's birth defect may have been caused by Paxil.




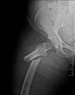 Fosamax, Boniva, Actonel, Aredia, and Zometa - medication prescribed to treat osteoporosis - are linked to femur fractures after 3 years of use. If you took any of this medication (or any combination thereof) and suffered a femur fracture,
Fosamax, Boniva, Actonel, Aredia, and Zometa - medication prescribed to treat osteoporosis - are linked to femur fractures after 3 years of use. If you took any of this medication (or any combination thereof) and suffered a femur fracture,  Mesh used during surgery to repair the vaginal wall has caused women to experience mesh erosion into the vagina, prolapse, pain during intercourse, incontinence, bowel or bladder perforation, bleeding and serious infection. We're now accepting clients with claims against AMS, Bard, Boston Scientific, Ethicon, and Johnson & Johnson. If you took have suffered any of these side effects,
Mesh used during surgery to repair the vaginal wall has caused women to experience mesh erosion into the vagina, prolapse, pain during intercourse, incontinence, bowel or bladder perforation, bleeding and serious infection. We're now accepting clients with claims against AMS, Bard, Boston Scientific, Ethicon, and Johnson & Johnson. If you took have suffered any of these side effects, 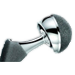 A growing number of metal-on-metal artificial hip implants are being recalled due to early failures and some patients suffering from metallosis - metal debris released from the hip compontents inflame the surrounding tissue and enter the bloodstream, causing elevated levels of cobalt and chromium. We're now accepting clients with claims aginst Biomet, DePuy, Stryker, Smith & Nephew, Wright, and Zimmer. If you have (or had) one of these devices, we can help to protect your legal rights. To contact us,
A growing number of metal-on-metal artificial hip implants are being recalled due to early failures and some patients suffering from metallosis - metal debris released from the hip compontents inflame the surrounding tissue and enter the bloodstream, causing elevated levels of cobalt and chromium. We're now accepting clients with claims aginst Biomet, DePuy, Stryker, Smith & Nephew, Wright, and Zimmer. If you have (or had) one of these devices, we can help to protect your legal rights. To contact us, 