Actos Bladder Cancer Lawyers - Nationwide
 Our Actos lawyers at Doyle Law are now reviewing cases involving patients who took the diabetes drug Actos® for more than one year and developed bladder cancer. Actos is manufactured, distributed and marketed by Takeda Pharmaceudicals around the world. It is co-marketed in the United States by Eli-Lilly and Company, one of the largest pharmaceutical companies in the world.
Our Actos lawyers at Doyle Law are now reviewing cases involving patients who took the diabetes drug Actos® for more than one year and developed bladder cancer. Actos is manufactured, distributed and marketed by Takeda Pharmaceudicals around the world. It is co-marketed in the United States by Eli-Lilly and Company, one of the largest pharmaceutical companies in the world.
Actos is a prescription medication used with diet and exercise to improve blood sugar or glucose control in adults with type 2 diabetes. Actos, generically known as pioglitazone, is an oral diabetes drug prescribed to help control Type 2 diabetes in patients whose conditions are not adequately controlled by diet and exercise alone. The drug belongs to a class of drugs called thiazolidinedione drugs and is intended to lower blood glucose levels and increase the body’s sensitivity to insulin.
Actos Side Effects
Actos causes bladder cancer.
The Food and Drug Administration (FDA) has warned that there may be a link between long-term use of Actos and bladder cancer. Recent studies indicate that Actos users are significantly more at risk of developing bladder cancer when compared to non-users.
Takeda Pharmaceuticals has updated their label warnings to include bladder cancer. Specifically, the label now states, "[p]reclinical and clincal trial date, and results from an observational study suggest an increased risk of bladder cancer in pioglitazone users. The observational data further suggest that the risk increases with duration of use. Do not use in patients with active bladder cancer. Use caution when using in patients with a prior history of bladder cancer."
What causes bladder cancer?
- Tobacco smoking is the main known contributor to urinary bladder cancer
- Chemotherapy and radiation therapy
- Long-term use of the chemotherapy drug Cytoxan which can irritate the bladder and increase the risk of bladder cancer. People taking this drug are often told to drink plenty of fluids to help
- Excessive grilled and/or barbequed meat and fat
- Occupational exposure to carcinogens such as bus drivers, motor vehicle mechanics, rubber workers, leather workers, blacksmiths, machine mechanics, gardeners/landscapers, farm workers, and hair dressers
- Genetic
- Toxic substances such as asbestos, chlorinated solvents, combustion, creosote, diesel engine exhaust, dust, leather, mineral oils, pesticides, paint, dyes in textiles/ hair dyes/printing, polycyclic aromatic hydrocarbons – byproducts of fuels burning
- Not drinking enough water
- Certain medications, such as the diabetes drug Actos, manufactured by Takeda Pharmaceuticals, have also been associated with causing bladder cancer.
Actos History
Takeda Pharmaceutical Company manufactures Actos. Launched in 1999, Actos has become the best-selling diabetes drug in the world with $4 Billion in sales during the 2008 fiscal year alone. Although Actos is manufactured by Takeda Pharmaceutical Company Limited, it is co-marketed in the U.S. by another industry giant, Eli Lilly and Company (the world’s 10th largest pharmaceutical company).
In June 2011, the FDA issued an Actos warning to doctors and patients that "use of the diabetes medication Actos (pioglitazone) for more than one year may be associated with an increased risk of bladder cancer." The FDA also stated that the Actos label and patient Medication Guide will be changed to reflect the potential risk of bladder cancer. This announcement was based on the FDA’s review of an ongoing, ten-year study revealing a disproportionate risk of developing bladder cancer with Actos use. The study reports that there may be a 40 percent increased risk of bladder cancer among those take Actos for more than one year.
Some countries have taken a more aggressive approach to limiting the sale and prescription of Actos. Following the release of a French study showing a possible increased risk of bladder cancer with Actos use, both France and Germany suspended sales and prescriptions of the drug.
According to the FDA website, the FDA will continue to monitor data from the ten-year study and will conduct a comprehensive review of the results from the French study. The Agency will also provide updates to the public when more information becomes available.
Alabama Actos Bladder Cancer Lawsuits
The Alabama Actos Attorneys at the Doyle Law have a proven track record of success in helping our clients obtain significant compensation for injuries resulting from the use of dangerous medications - like Actos.
Our experienced team of legal professionals and Actos lawyers has been helping dangerous drug injury victims for years. We are dedicated to achieving real justice for the clients we serve can assure them that we will work diligently to achieve a satisfactory result.

Contact Alabama Actos Bladder Cancer Attorneys
Filing an Actos bladder cancer lawsuit can help patients and their families affected by the Actos diabetes medication recover the financial compensation necessary to pay for expensive treatment and long term care. If you or a loved one developed bladder cancer after your actos use, you may have a defective product claim against Takeda Pharmaceuticals and Eli-Lilly. Call our Birmingham, Alabama, offices today for a free consultation. Our office number is (205) 533-9500. Do not wait another day and risk losing your claim.
Alabama has a two year statute of limitations on filing certain product liability and personal injury claims. It takes time to acquire medical records and draft pleadings, so please do not delay in contacting us if you have any concern that your bladder cancer may have been caused by Actos.




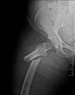 Fosamax, Boniva, Actonel, Aredia, and Zometa - medication prescribed to treat osteoporosis - are linked to femur fractures after 3 years of use. If you took any of this medication (or any combination thereof) and suffered a femur fracture,
Fosamax, Boniva, Actonel, Aredia, and Zometa - medication prescribed to treat osteoporosis - are linked to femur fractures after 3 years of use. If you took any of this medication (or any combination thereof) and suffered a femur fracture,  Mesh used during surgery to repair the vaginal wall has caused women to experience mesh erosion into the vagina, prolapse, pain during intercourse, incontinence, bowel or bladder perforation, bleeding and serious infection. We're now accepting clients with claims against AMS, Bard, Boston Scientific, Ethicon, and Johnson & Johnson. If you took have suffered any of these side effects,
Mesh used during surgery to repair the vaginal wall has caused women to experience mesh erosion into the vagina, prolapse, pain during intercourse, incontinence, bowel or bladder perforation, bleeding and serious infection. We're now accepting clients with claims against AMS, Bard, Boston Scientific, Ethicon, and Johnson & Johnson. If you took have suffered any of these side effects,  A growing number of metal-on-metal artificial hip implants are being recalled due to early failures and some patients suffering from metallosis - metal debris released from the hip compontents inflame the surrounding tissue and enter the bloodstream, causing elevated levels of cobalt and chromium. We're now accepting clients with claims aginst Biomet, DePuy, Stryker, Smith & Nephew, Wright, and Zimmer. If you have (or had) one of these devices, we can help to protect your legal rights. To contact us,
A growing number of metal-on-metal artificial hip implants are being recalled due to early failures and some patients suffering from metallosis - metal debris released from the hip compontents inflame the surrounding tissue and enter the bloodstream, causing elevated levels of cobalt and chromium. We're now accepting clients with claims aginst Biomet, DePuy, Stryker, Smith & Nephew, Wright, and Zimmer. If you have (or had) one of these devices, we can help to protect your legal rights. To contact us, 